FORM 1-PHYSICS-STRUCTURE AND PROPERTIES OF MATTER
Structure of Matter
Elasticity
Adhesion and Cohesion
Surface Tension
Capillarity
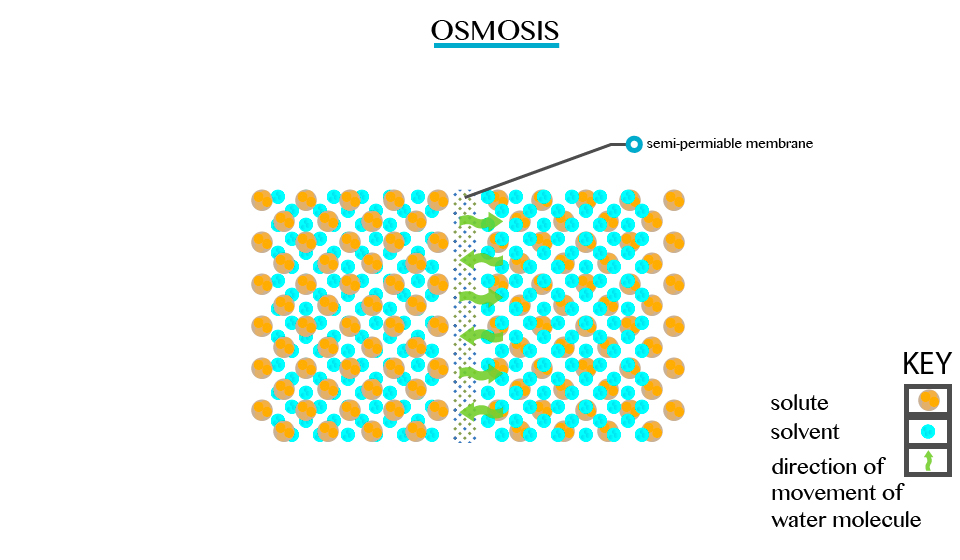
Osmosis
The Concept of Osmosis
Explain the concept of osmosis
Defined as the movement of a solvent from a region of low concentration through semi permeable membrane.
Particles will diffuse through the membrane in an attempt to equalize the concentration on either side. E.g. two solutions of different concentration separated by a semi permeable membrane. The membrane is permeable to the smaller solvent molecules but not to the larger solute molecules. Osmosis stops when the concentration becomes the same on either side of the membrane.
Osmosis stops when the concentration becomes the same on either side of the membrane.
Applications of Osmosis in Daily Life
Identify the applications of osmosis in daily life
Applications of osmosis in daily life:
- Control the movement of water and nutrients in and out of the cell.
- Filtration processes.
- Removal of harmful ingredients from dinking water.
- Removing salt from seawater so as to make it suitable for drinking and for other domestic uses.






