FORM 1-PHYSICS-STRUCTURE AND PROPERTIES OF MATTER
Structure of Matter
Elasticity
Adhesion and Cohesion
Surface Tension
The Concept of Surface Tension
Explain the concept of surface tension
While you may not be able to walk on water, water stride does. This is due to the property of liquid, which is known as surface tension.
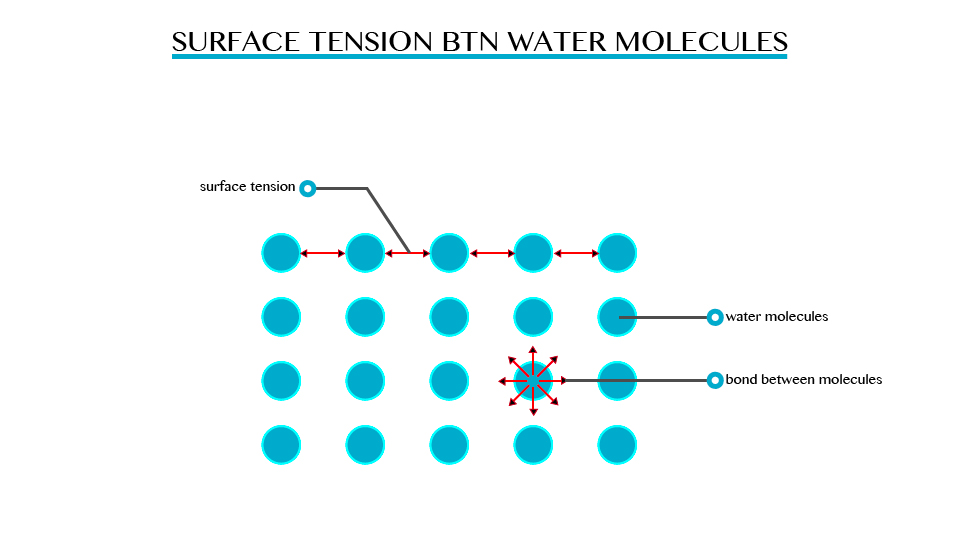
Surface tension is the ability of the molecules on the surface of a liquid to attract and stick to each other allowing them to resist an external force. Surface tension enables insects such as water strides and mosquitoes to walk on water. It allows small objects even metallic ones such as needles and razor blades to float on the surface of water.
Surface tension is a resultant attractive force between molecules in a liquid. The molecules below the surface liquid have forces of attraction between neighbouring particles. However molecules at the surface have no neighbouring molecules above them. This makes them have stronger attractive force than their nearest neighbours on the surface.
However, when some detergent is added to water, the same objects sink to the bottom of the trough. This means that the detergent interfered with the surface of the liquid so decreasing the tension of the water surface.
Detergents are example of surfactants. A surfactant is a substance that reduces the surface tension of a liquid.
Note: the term surfactant is an aerogun for surface-active agent.
Surface tension is affected by the following
- Nature of the liquid
- Contamination/impurities
- Temperature
Application at surface tension:
- In extraction of impurities dating laboratory process.
- Surfactants are also used to make emulsion of liquid like oil and water.
- In cleaning action of soap.
Applications of Surface Tension in Daily Life
Identify the applications of surface tension in daily life
Application at surface tension
- In extraction of impurities dating laboratory process
- Surfactants are also used to make emulsion of liquid like oil and water.
- In cleaning action of soap
Capillarity
Osmosis






