FORM 1-PHYSICS-STRUCTURE AND PROPERTIES OF MATTER
Structure of Matter
State of matter is defined in terms of the phase transitions which indicate the change in structure and properties. Solids, liquids and gases all are made up of microscopic particles. The behavior of all these particles also varies in three phases.
The Concept of Matter
Explain the concept of matter
Matter is anything, such as a solid, liquid or gas, that has weight (mass) and occupies space. For anything to occupy space, it must have volume.
The Particular Nature of Matter
Justify the particulate nature of matter
Matter is made up of tiny particles. The particles are atom or molecules, examples of substances, which are made up of atoms, are: gold, copper, Argon and silver; and those made up of molecules includes oxygen, water and ammonia.
- In solid, storm’s attractive forces hold molecules together so that they are not free to move but they can only vibrate about their mean positions.
- In liquids there are weak forces of attraction between molecules therefore the molecules are free to move randomly. The distances between molecules in liquids are therefore are larger than in solids.
In case of gases the molecules experience very weak forces of attraction and hence they are free to move randomly filling the whole space of the containing vessel. The distances between molecules in gases are comparatively greater than those in solids and liquids as shown in the figure above.
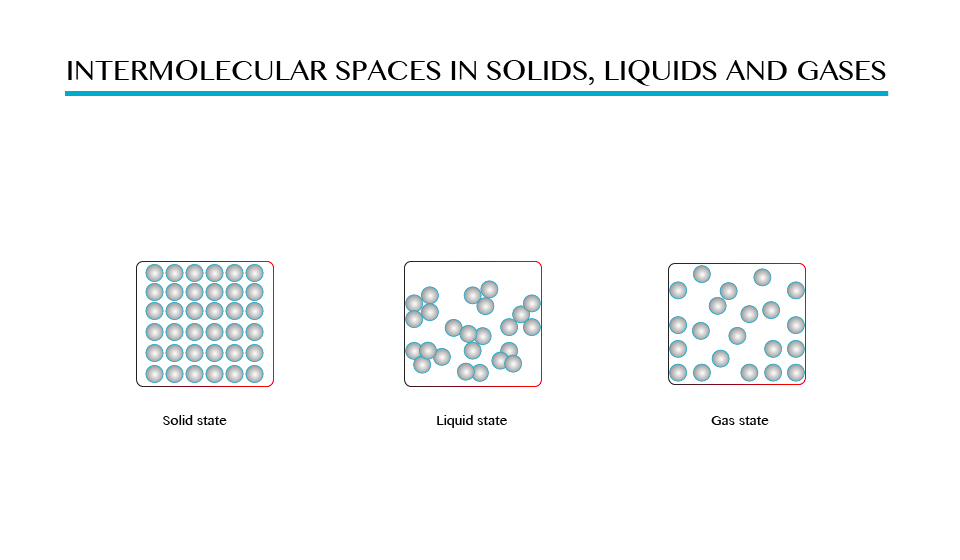
Demonstration to show the intermolecular space in solids, liquid and gases.
The Kinetic Theory of Matter
Explain the kinetic theory of matter
Generally, when solid particles are placed in the source of lead the particles tends to move from hot area to cold areas. These particles move because it gains energy that called it Kinetic energy.
Kinetic theory of matter sometimes attempts to explain how properties of gases like pressure, temperature and volume remain in constant motion.
There are three main parts of the Kinetic theory of matter. This includes:
- Matter is made up of tiny invisible part.
- Matter comes in different sizes.
- There is a point that the smallest particles of matter can be the fastest.
Therefore kinetic theory of matter states, “All matter is composed of small particles”Or “Particles of matter are in steady motion and that all impacts between the units of matter are completely elastic”
Three States of Matter
Classify three states of matter
There are three states of matter, namely:
- Solid state
- Liquid state
- Gaseous state
Solid state is the state of matter, which include solid materials, in which the intermolecular force between molecules are greatest and distance between molecules is small. Examples of solid state are wood, iron, etc.
Liquid sate is the one of the state of matter in which the intermolecular forces are low compared to solid state, there is greater distance between one molecule and another. See on figure 1.0 (b) examples water, soda, kerosene, and petroleum.
Gaseous state is the state of matter in which there is no intermolecular forces between molecules hence molecules are free to move from one place to another examples of gases are hydrogen, oxygen, carbon dioxide gas.
Difference between solid state, liquid state and gaseous state of matter
| Solid state | Liquid state | Gaseous state. |
| It concerns with solid matter | It concerns with liquids/ fluids matter | It concerns with gases |
| Have high intermolecular | Low intermolecular force | No intermolecular force |
| No distance between molecules | There is little distance between molecules | Molecules are far from each other |
| Good examples are iron materials, woods etc. | Good examples are water, soda, kerosene and petrol | Good examples are oxygen and hydrogen |
Rownian Movement
According to Robert Brown: Brownian movement refers to the irregular motion of tiny particles suspended in fluid (liquid organs). Consider the demonstrationbelow
Robert Brown, an English Botanist, powered some pollen grain in water and observed that particles floating in the water were darting about.
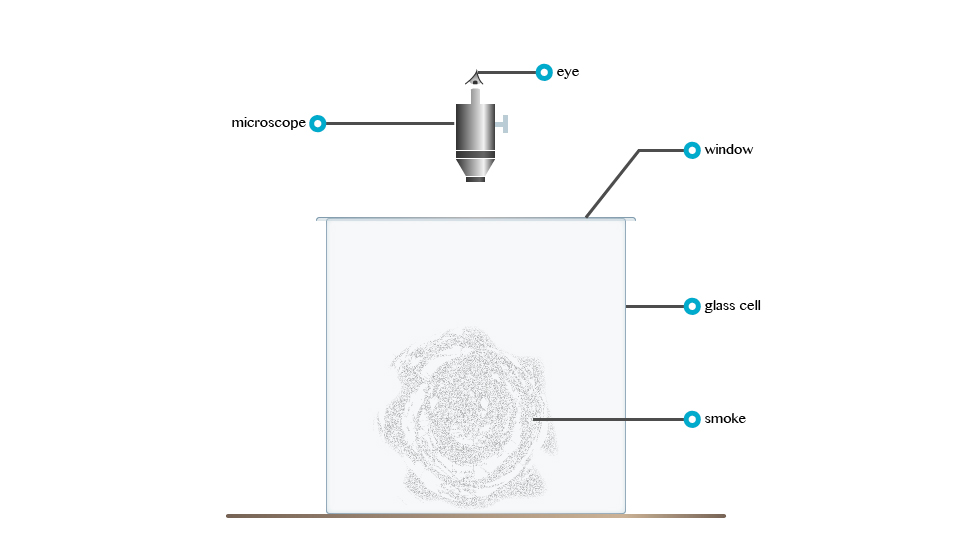
The irregular motion of tiny particles suspended in a fluid (fluid or gas) is called Brownian movement.
The tiny particles dart about because liquid molecules that are in state of motion bombard them.






